1- INTRODUCTION
The Institut De Recherche En Santé De Surveillance Épidémiologique Et De Formation (IRESSEF), through funding from the U.S. Centers for Disease Control and Prevention (U.S. CDC), is working to identify a partner that is based in and operating in South Africa to support the implementation of the scope of work for this Revised Strategy for the Implementation of the Integrated Clinical Laboratory Interface (CLI) and Rapid Test Continuous Quality Improvement (RTCQI) Program (CLI/RTCQI).
1.1 Organizational Scope
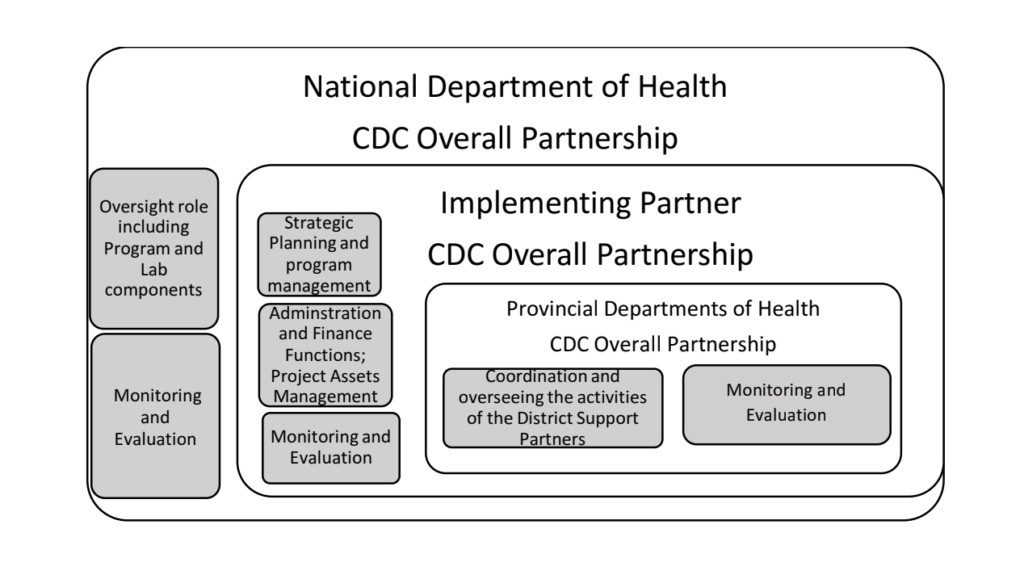
The revised project will be implemented through an appointed dedicated CLI/RTCQI country partner in collaboration with the National Department of Health (NDOH), Provincial Department of Health (PDOH) in PEPFAR supported provinces and District Support Partners (DSPs). The appointed overall project partner scope of work will focus on:
a) Oversee the implementation of the CLI/RTCQI program on behalf of the National Department of Health through PEPFAR support.
b) Provide overall administrative functions of the project including human resources, finances, procurement, overseeing the implementation of the CLI/RTCQI program in targeted HIV high burden health facilities in conjunction with the NDOH and PDOH in PEPFAR supported provinces whilst providing support to non-targeted health facilities.
c) Manage and/or oversee the overall implementation of the CLI/RTCQI program in HIV program and related programs with focus on targeted HIV high burdened districts and health facilities to ensure interventions have impact on HTS and the standard requirements for quality HIV diagnostics are maintained.
d) Assist the Department of Health with the review and implementation of the national training curriculum for HIV rapid testing to close gaps in training of HTS Counsellors, Enrolled Nurses and Professional nurses in conjunction with NDOH, PDOH, DSP’s in PEPFAR supported provinces.
e) Review and implement competency certification program for test providers including HTS counsellors, enrolled and professional nurses in conjunction with NDOH, PDOH in PEPFAR supported provinces and
DSP’s.
f) Support and oversee participation of targeted testing sites in Proficiency Testing (PT) and Quality Control (QC) programs.
g) Manage the logistics of project goods, materials and supplies including supporting the distribution of PT and IQC serum panels and PT feedback reports in conjunction with the National Health Laboratory Service (NHLS) and PDOH in PEPFAR supported provinces.
h) Manage Assets, including new and existing equipment. NDOH to ensure smooth transfer of assets to the new project partner.
i) Work with established Technical Working Group(s) in NDOH, PDOH including DSPs on areas of strategic importance to HIV program and related disease programs.
1.2 Proposed Operations Structure
The following are key stakeholders:
• National Department of Health
• Provincial Department of Health
• Project Partner
• District Support Partners
• CDC SA Overall Partnership
2- OBJECTIVE
The revised CLI/RTCQI implementation strategy builds on previous experiences and is aimed to address existing barriers and improve impact of the program on the ground and consequently a quality-assured and sustainable HIV Testing Services (HTS).
3- Number of Contractor: One- Three (1-3)
Preference will be given to organizations based and operating in South Africa.
4- Scope of Work:
a) Provide overall administrative and supervision of day-to-day operations including hiring, project staff orientation, training, monitoring and evaluation of staff performance to ensure efficient and seamless project operation.
b) In conjunction with NDOH and PDOH in PEPFAR supported provinces, develop project scope including planning, organization of resources, work plans, project objectives, tasks, calendar of activities, timelines and track progress.
c) Oversee and support the distribution of materials including PT and IQC serum panels, PT feedback reports.
d) Manage and/or oversee the implementation of the CLI/RTCQI components including training, tester competency and certification, external quality assessment viz. SPI-RT external site assessment.
e) Develop and implement coaching/mentorship program for test providers including HTS counsellors, Data Capturers, Enrolled and Professional Nurses in selected high-volume facilities in collaboration with the DSPs Lab Coordinators and CLI/RTCQI Coordinators.
f) Coordinate stakeholder meetings to facilitate planning, calendar activities and periodic feedback report meetings at national, provincial and district levels.
g) Support and oversee the DSPs implementation of CLI/RTCQI activities, provide oversight and monitor progress and integrity of implementation.
h) Implement an intensive annual external assessment to meet the requirements of PEPFAR reporting indicators.
i) Manage project funds including budgeting, forecasting, utilization of funds, expenditure and keep financial records including provision of financial reports.
j) Manage procurement processes relating to the provision of goods, supplies, materials and services for smooth running of the project.
k) Manage tender processes for acquisition of goods, supplies, materials and services when it is required.
l) Develop program support materials including revised activity standard operating procedures or job aides to facilitate and make simpler task performance at the testing sites.
m) Provide capacity building of the Operational Managers/Facility Supervisors, HTS/TB/STI Program Managers and Laboratory Area Managers gradually establishing sustainability plans.
5- Deliverables
a) Hire 8 people to be seconded to the provincial Department of Health in PEPFAR supported provinces, and provide all HR related support to them
b) A detailed project scope including organization of resources, work plan, project objectives, narratives, activities timelines.
c) A detailed project budget, forecasting and financial reports detailing the utilization of funds and expenditure status.
d) Number of certified tester competency assessors in each PEPFAR supported province
e) Number of testers Certified in each PEPFAR supported province
f) External site assessment plan and number of completed assessments.
g) Approved tasks support materials including standard operating procedures or job aides.
h) Participate and contribute towards the approval of training and competency assessment materials
through the relevant bodies e.g. Regional Training Centres (RTCs).
i) . Hire an IT person that will provide IT support for all ECHO sites for CLI/RTCQI trainings in the Regional Training Centers.
The national project partner will work in collaboration with NDOH, PDOH and DSPs in order to achieve quality-assured and sustainable continuous quality improvement for HIV rapid testing in health facilities to:
a) Facilitate and promote ownership of the programme by the National and Provincial Departments of Health.
b) Leverage and strengthen existing local quality assurance resources and capacity established in previous years.
c) Promote the culture of continuous quality improvement.
d) Identify key areas in CLI and RTCQI that requires quality improvement (QI) capacity building through empowering operational levels in order to improve uptake and facilitate sustainability.
e) Strengthen the integration of QI networks across the country.
6- Timeline
October 1, 2019 to September 30, 2020
Total Approximate Budget: Not to exceed USD 1,400,000.00
It is important to note that the resulting contract will included phase implementation and funding based on
the expected timeline and deliverables.
7- APPLICATION COMMENTS
A potential contractor may compete for the project in a single application. Final selection for the project will be based on technical capacity and other considerations as deemed necessary by the scope of work. Proposed costing
will also be considered as an additional basis for selection.
IRESSEF shall enter into a contract with the selected contractor. Duration of contracts shall be up to a maximum of 12 months beginning on October 1, 2019.
8- INSTRUCTIONS FOR SUBMITTING PROPOSALS
A. The format of the submission, in response to this RFP, must include, but is not limited to, the following:
i. Description of project background and objectives
ii. How the Respondent proposes to accomplish the functional area (s). Include specific objectives and activities that will be done under each objective
iii. Technical Approach: Activities that will be done to cover the proposed scope of work and achieve relevant objectives
iv. Monitoring and evaluation plan: Include a feasible and appropriate monitoring and evaluation plan; including expected outcome indicators
v. Organizational Capabilities and Experience: A description of the respondent’s work history (experience) with similar projects in the past five (5) years (Capability Statement).
vi. Description of key individuals and staff qualifications and experience. Indicate how these qualifications and experience relate to the proposed activities.
B. Completed proposals shall consist of typewritten pages utilizing 12” font typing. A maximum of 10 pages for the proposal is allowed
C. The authorized individual representing the Respondent will sign and date the proposal cover sheet. The signatory agent’s printed name, title, name of the organization, address, phone and fax numbers and email
address must be provided. Failure to provide a signed copy of the affirmation statement below will be cause for the proposal not to be considered. I affirm that the information within this proposal, to the best of my knowledge, is true and accurate. Further, I am duly authorized to sign and submit this proposal on behalf of this agency. I fully affirm and understand that failure to meet the requirements of this proposal at the submitted price may result in my organization’s contract being terminated.
D. Include at least two (2) current references and their contact information from organizations that have used your services within the last twelve (12) months.
Send your completed application by email to Mimi Traoré-Mané at maimouna.traore@iressef.org on or before October 14th, 2019 at 5:30pm East African Time.
9- EVALUATION AND AWARD PROCESS
A committee formed from staff and outside experts will evaluate the applications based on preset standards relevant to the specific project and its correlation with the RFP objectives. An evaluation matrix with assigned weighted numerical values will be used to rate each applicant. The following represents the criteria that will be used to the assessed feasibility of each Respondent.
Knowledge of the project needs and objectives 10%
Organizational Capabilities and Experience 25%
Technical approach 25%
Monitoring and Evaluation plan 20%
Qualification and expertise of the proposed key staff(s) 20%
Budget (reasonableness and total value) 0%
Applicants must be entities based in South Africa and should have experience working with and supporting laboratories and health systems in the country. IRESSEF reserves the right (but is not under obligation to do so) to enter into discussions with one or more Respondents in order to obtain clarifications or additional details, to suggest service delivery refinements in the proposal or other aspects of the proposal, or to negotiate the cost proposal.
Each Respondent submitting a proposal will be notified in writing or via e-mail of the decision concerning their proposal. Should your organization be recommended for acceptance, the contract shall be effective on the contract execution date. All work must be scheduled and completed within the contract period timeframe. Any modifications or extensions must be negotiated in advance and submitted to IRESSEF for review and approval. The selected Respondent’s proposal, and any subsequent material submitted in response to requests for additional information, will become the basis of contractual agreements with said Respondent.
10- CONTRACTUAL TERMS AND CONDITIONS
Responses must be in accordance with the guidelines as specified in this RFP. This RFP does not commit IRESSEF to accept any proposals submitted, nor is IRESSEF responsible for any costs incurred in the preparation of responses to this RFP. The detailed itemized budget must be submitted in U.S. dollars and will be evaluated in terms of best value to IRESSEF.
IRESSEF reserves the right to delay, amend, reissue or cancel all or part of this RFP at any time without prior notice.
IRESSEF will be under no obligation to reveal or discuss with any Respondent on how a proposal was assessed, or to provide any other information relative to the selection process. Respondents whose proposals are not selected will be notified by email and shall have no claim whatsoever for any kind of compensation.

